Phage Comparative Genomics Tools
Overview
QuestionsObjectives
Requirements
Time estimation:
Agenda
In this tutorial, you will find:
Background
- DNA Sequence Comparisons
- Protein Sequence Comparison
Workflow
Background
The functional annotation workflow has been run and gene prediction has at least started on your novel phage genome. Now it is time to start looking at relationships between the novel phage and other known phages. The best way to do this is to compare the novel phage genome to sequences that are already deposited in the sequence databases. This comparison can be executed in two ways:
- Compare the entire DNA sequence of the novel phage to the DNA sequences of other organisms
- Compare the protein sequences of all the genes in the novel phage genome to the protein sequences of other organisms
As part of the functional workflow, BLASTp (for proteins) and BLASTn (for nucleotide sequences) have already been run; thus, both of these kinds of comparisons have already been made. The BLAST results will be compared against the NCBI NR and NT databases for proteins and DNA, respectively. These are the most current and comprehensive databases as they reflect the internationally shared INSDC database.
A Brief Center for Phage Technology Precedent
An overview of how the CPT currently organizes and classifies genomes is provided in this publication.
DNA Sequence Comparisons
Because the triplet code that encodes protein sequences is degenerate (as in, there are multiple possible triplet codons for most amino acids), a DNA sequence can drift and still encode the same protein sequence by accumulation of silent mutations. This means that DNA sequences encoding similar proteins can diverge at a relatively high rate, thus DNA sequence comparisons are only particularly useful for organisms that are closely related. Between related organisms, DNA sequence comparisons can provide good overall parallels, as this single analysis can demonstrate both conservation of DNA sequences and genome synteny (the order of genes in the genome). Additionally, high conservation of DNA sequences automatically means that proteins encoded by that sequence must also be similar. Once DNA sequence similarity drops below ≈ 30%, it is no longer very useful for comparisons.
Protein Sequence Comparisons
Comparison of organisms by protein sequence is much more sensitive, as there is stronger pressure to conserve a protein sequence so that the protein will retain its function. Phage genome organization is considered to be modular, meaning that individual genes or groups of genes can be shared across otherwise very different phages. It is not unusual for two phages to be very similar across the genome with only particular genes - such as phage tail fibers - being different.
Modular Phage Gene Examples
Comparing phages lambda and P22, one will see that they share similar integration and lysogen genes, but different morphogenesis genes (siphophage versus podophage, respectively). Comparing phages lambda and T1, one will see that they have related morphogenesis genes, but different modules for control of replication and lysis.
Workflow
The current comparative genomics workflow is set up to perform TaxID-restricted BLAST jobs against all phages (see workflow BLAST job for current list). Because of this restriction, the BLAST jobs are relatively quick. The BLAST results will then be filtered to display top hits, and the nucleotide hits will be compared using Mauve to calculate percent identity.
Restriction by TaxID
Since this workflow is meant to uncover the closest phage relatives, the BLAST jobs are restricted by all known phage NCBI TaxIDs (including Caudovirales, Leviviridae, Cystoviridae, Inoviridae, Microviridae, Corticoviridae, Tectiviridae, Plasmaviridae, Gammasphaerolipoviruses, unclassified bacterial viruses, and unclassified dsDNA phages). To instead compare only to sequenced (though not necessarily annotated) prophage, change the BLAST job TaxID restriction to 2 (the NCBI TaxID for Bacteria).
- Open CPT Galaxy (CPT Public Galaxy, CPT TAMU Galaxy), and find the history that contains the results of the functional workflow for the desired phage genome.
- Locate the genome FASTA file and the protein multi-FASTA file (likely called Fasta translate).
- Create a new history by clicking on Create New (top right).
- Locate the two datasets needed (Translated proteins and your FASTA sequence) that you need, then click and drag them over to your new history to copy the datasets there. As you are dragging toward the active history (the new one that you just created), a box will appear that says “Drag datasets here to copy them into the current history”. Drop the datasets in that box and soon a green box with your new dataset should appear.
- After copying all necessary datasets, return to the main Galaxy analysis interface by clicking Done in the top left.
Can’t find the input dataset?
When workflows are run in Galaxy, they often hide the output of their tools to give a clean and readable history with only relevant datasets shown. Sometimes a later analysis will require that dataset and it must be unhidden so that it can be used as input for a tool. At the top of the history, click on the “hidden” hyperlink to see all workflow outputs that have been hidden. Scroll down and find the desired dataset. Click “Unhide it” on this dataset and it will become available for analysis. If preferred, click “hide hidden” at the top of the history to re-hide the other datasets to prevent clutter.
- At the top of the web page, click on the Shared Data drop-down menu and select workflows.
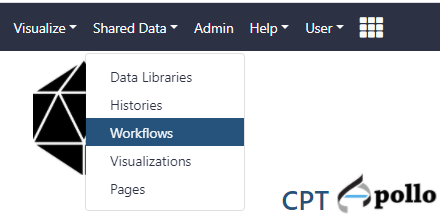
- Find the most recent version of the “Phage comparative genomics (v#) workflow,” where # indicates the most recent version of this workflow. Click on the drop-down menu for that workflow and select “Import.” After this, a green box containing a message will appear to inform the user of a successful import. ALternatively, the workflow can be run directly from here, without importing, by clicking on “Run”.
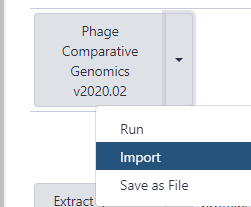
- Once imported, one can click on “start using this workflow” within the message box to be brought to the page containing all of the user’s imported workflows. Find the Phage comparative genomics workflow, click the drop-down menu, and select “Run.”
- The parameters for the workflow will then load in the center Galaxy panel. The inputs that the user needs to adjust are as follows:
- Step 1. The Input phage DNA sequence (FASTA) will be the FASTA dataset (raw nucleotide for genome)
- Step 2. The Export protein-coding genes for comparison (Galaxy Version 19.1.0.0) will be the GFF3 file
When Retrieving Data from Apollo into Galaxy
When the Retrieve Data tool is used to import data from Apollo into a Galaxy history, the resulting file is a combined GFF3 + FATSA which must be split into separate files before the Comparative Genomics workflow is run. Search for the Split GFF3 + FASTA into separate parts tool in the Tools pane, then run this tool on the combined file to generate the seprated files. When running the workflow, make sure that the input GFF3 and FASTA files are the split GFF3 and FASTA files and not the “Annotations and Sequence from Apollo” file.
Note that…
The dataset numbers will be different for each user. Workflow versions also change regularly.
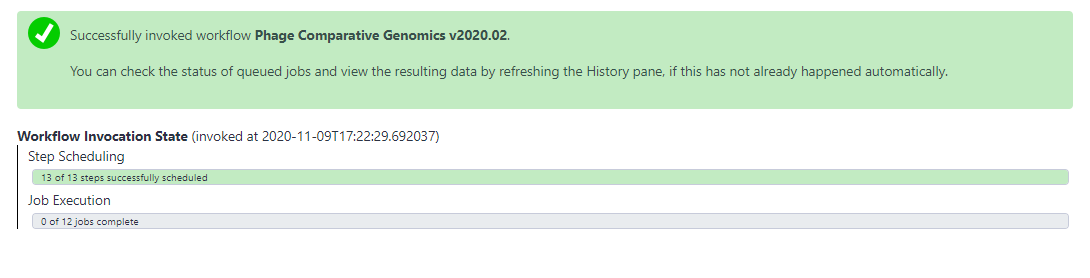
- Once the inputs have been selected, click “Run workflow” at the top of the page to execute the workflow. A message in a green box will appear to inform the user of a successful invocation of the phage comparative genomics workflow and beneath that it will show you the Workflow Invocation State as the jobs are scheduled then run. From here, wait until all of the steps have completed and their datasets have turned green.
Issues with Workflow
If the dataset turns red in the history column, click on it to expand it. Report the bug by clicking on the bug icon in the bottom left-hand corner of the expanded dataset.
When the workflow has completed running, you can investigate what phages may be closely related to the one being annotated by looking at the tool outputs. This workflow should have produced four new datasets in the history column: Top BLASTn hits, Top BLASTp hits, MIST v3, and Percent nucleotide identity from Mauve XMFA.
Look at the Top BLASTn hits data by clicking the eye symbol. A small table will list the top 5 most similar organisms to the phage in question at the DNA level, as determined by BLASTn. If a phage in the database has high similarity at the DNA level to the phage in question, it is worthwhile to line up the genomes and visualize just how related they are; an easy way to do this is via a comparison method called dot plot, which will visualize a pairwise comparison of the two sequences.
The results of a dot plot analysis can be seen by clicking on the eye symbol in the MIST v3 dataset. This will be the results of pairwise dot plots of the phage in question against the 5 closest-matching genomes. Phages that are related and syntenic will produce a discrete diagonal line in the dot plot. As DNA sequence similarity decreases, the line will become fainter and patchier until it is no longer visible.
Utilizing relationships and similarity at the protein level, the Top BLASTp hits dataset displays a table of the top 20 most related organisms based on the number of similar proteins found by BLASTp. This tool searched through your BLASTp results against the NCBI nr database and retrieved the organisms that matched the highest number of proteins in your phage (an E-value < 1e-3). This number can be found in the last column of the table. Note that this table shows only the top 20 results. If the phage in question is part of a large cluster of similar phages (E.G.: T4-like phages), then T4 itself may not appear in the list; 20 other T4-like phages may be more closely related to the phage in question, and T4 may be only the 30th most related phage. The does not mean the phage in question is not T4-like, and it is up to the annotator to determine how the phage in question relates to other phages. See the box below for more additional investigations that may help.
Further sleuthing…
There are several ways that the annotator can use the output of these analyses to determine the closest type phage. Note that these will all be under the classification Caudovirales.
- Copy the phage name or number. Go to the NCBI Taxonomy browser and search the name. Click on the name you searched to analyze its lineage.
- Copy the phage name and search it at the ICTV Taxonomy. Find the closest related type phage under Order Caudovirales.
- Copy the phage name and search it at ViralZone. Find the closest related type phage. Not all phage are listed in ViralZone, so do not be alarmed if you have no results here.
Congratulations on successfully completing this tutorial!
Help us improve this content!
Please take a moment to fill in the Galaxy Training Network Feedback Form. Your feedback helps us improve this tutorial and will be considered in future revisions.
