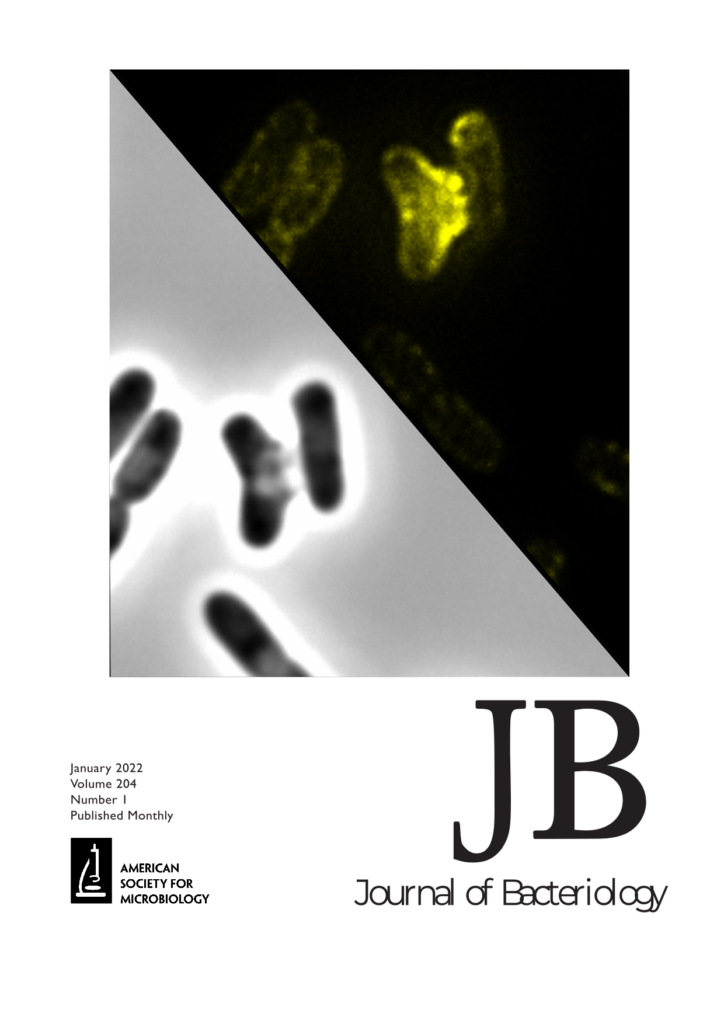
Recent work from Young lab made the cover of Journal of Bacteriology with their story on a new class of phage lysis proteins, the disruptins. The work is described in an special issue of Journal of Bacteriology on small proteins. The journal cover features a cell lysing due to the disruptin-encoding phage infection.
The featured work started a few years ago, when an undergraduate Beckman Scholar named Ashley Holt was funded to study how phage lyse host cells in the Young lab. Working under Dr. Jesse Cahill, she started looking for a missing protein in our understanding of molecular lysis mechanisms. The Young lab had shown that most phage require a unique class of proteins called spanins to complete efficient and timely lysis. However, lab alum Dr. Rohit Kongari had shown nearly 15% of phage genomes were missing spanin genes. If not using spanins, then how do these phage cross that outer membrane barrier? The search started in an E. coli phage isolated from Russian horse fecal samples.
Surprisingly, Ashley found that a small protein with uncanny similarity to mammalian cationic antimicrobial peptides provided outer membrane-disrupting capability. The new class of lysis protein was dubbed the ‘disruptin’. Additional team members, including co-first author Dr. Jolene Ramsey, showed the disruptin was strongly associated with the bacterial membranes that it compromises. Through a collaboration with members of Dr. Jeffrey Cirillo’s lab it was demonstrated that the disruptin is bacteriostatic and bactericidal when added to cells externally at concentrations similar to the classic antimicrobial peptide LL-37.
Interestingly, like most antimicrobial peptides, the disruptin shares little sequence similarity with proteins outside close relatives. Given the many phage whose lytic proteins are not identified or remain uncharacterized, this suggests additional phage using antimicrobial peptide-like molecules to complete lysis, or another mechanism, are yet waiting to be found.