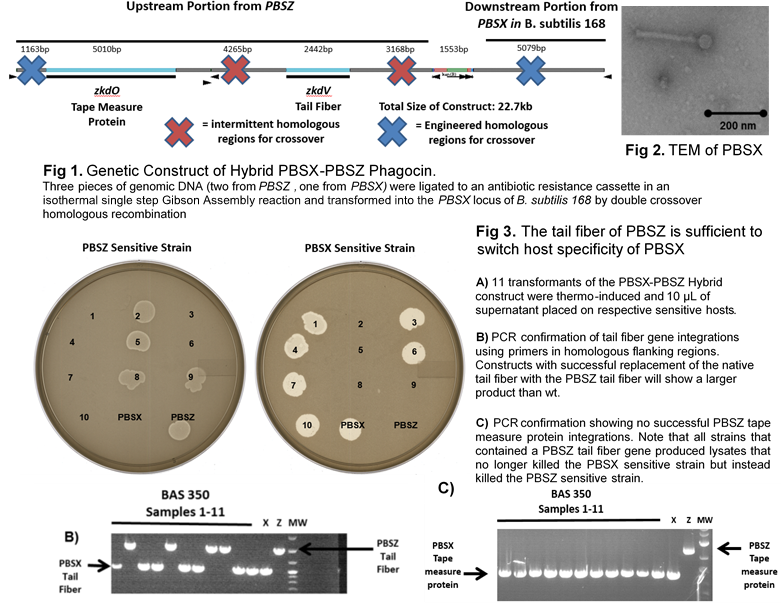
PBSX is a temperate bacteriophage mutant of Bacillus subtilis 168 incapable of infection while maintaining antimicrobial characteristics with unit efficiency. PBSX-like defective prophages, called phagocins, are induced by the SOS response of various Bacillus genera and function as antimicrobial agents capable of targeting species other than the host. Disease caused by dysbiosis of the human microbiome and antibiotic-resistant bacteria has promoted research for novel antimicrobials, such as bacteriophage, to address the problems of the indiscriminate killing of the microbiota by classical antibiotic approaches. Bioinformatics study of the PBSX-like bacteriophage mutants reveals nearly identical genomic make-up except in the regions containing the tape measure and tail fiber genes, implying relative modularity of the PBSX-like phagocins. In the native PBSX locus we demonstrate that the tail-fiber structural gene xkdV is responsible for targeting wall-teichoic acid components. Upon exchange of the specificity determining domain of the PBSZ tail fiber in the PBSX locus, PBSX acquires the host range of PBSZ. Here we present a PBSX system capable of host-range engineering as a docile approach to phage therapy. Our data show: a thermoinducible system for phagocin production as an antimicrobial, tail fiber exchange is sufficient for swapping specificity of PBSX killing ability, B. subtilis 168 allows for easy manipulation of the endogenous PBSX, and the PBSX locus can be optimized to produce a billion killing units/ml raw lysate. Future studies will focus on leveraging this adaptable antimicrobial as a specific tool for the remodeling of the human microbiota, specifically targeting those microbes that dominate the immature microbiota (Bifidobacterium longum, Lactobacillus mucosae, Streptococcus spp. and Clostridium spp.).
Center for Phage Technology: Home / Current Research Projects / PBSX phagocin: an antimicrobial with adaptable specificity in a modular framework