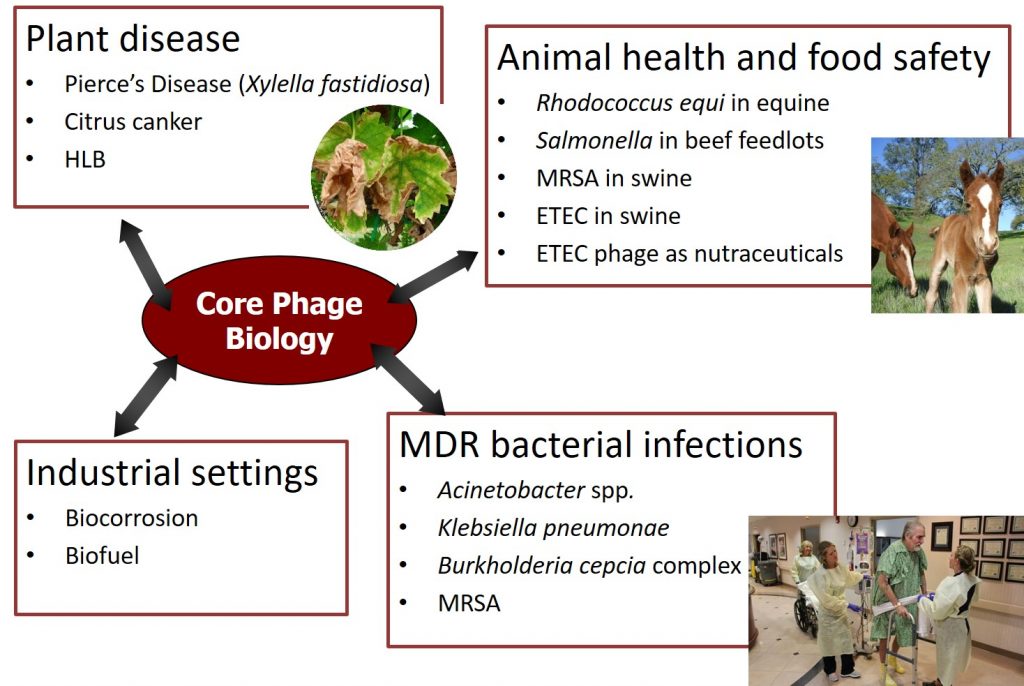
Phages hold great potential as natural antimicrobials. Supported by solid foundation of phage biology, we have investigated phages that infect the pathogens in various systems, including plant diseases, animal health and food safety, multi-drug resistant (MDR) bacterial infections in humans, as well as in industrial settings. Current major research areas are as follows.
Multi-drug resistant (MDR) bacterial infections in humans
- Acinetobacter and the Tom Patterson case
Acinetobacter baumannii is frequently isolated from infections in clinical settings and has high levels of acquired and intrinsic antibiotic resistance. Acinetobacter infections are of particular concern to active-duty military service members injured in combat. While on vacation in Egypt in 2015, Tom Patterson, a 68-year-old professor at the University of California San Diego, fell ill with a disseminated A. baumannii infection that was highly drug-resistant. Comatose and undergoing multiple organ failure, he was medevaced to the UCSD Medical School hospital in La Jolla, CA, where his condition continued to deteriorate, with no treatment options available. In desperation, his wife, Steffanie Strathdee, contacted Dr. Young at the CPT to recruit him to help her find phages to treat her husband.
In conjunction with US phage therapy companies, the US Navy MRC, and the international phage community, the CPT isolated and prepared a therapeutic phage cocktail, used to treat Dr. Patterson under FDA expanded access (eIND) protocols. Over the course of several days, phages were administered via percutaneous catheters, and a second cocktail prepared by the US Navy group was administered intravenously. Within 48 hours of treatment, Tom woke from his coma. Phage treatment continued, and eventually he went on to make a complete recovery from the infection. A case report detailing this treatment was published in 2017 in Antimicrobial Agents and Chemotherapy. A memoir of this story written by Tom Patterson and Steffanie Strathdee, The Perfect Predator: A Scientist’s Race to Save her Husband from a Deadly Superbug was released in 2019.
As a result of this trial, the CPT is now collaborating with the Center for Innovative Phage Applications and Therapeutics (IPATH) at UC San Diego in isolating and formulating therapeutic phages for eIND applications against a range of bacterial species and diseases.
Related publications:
Genomic and Biochemical Characterization of Acinetobacter Podophage Petty Reveals a Novel Lysis Mechanism and Tail-Associated Depolymerase Activity. (PMID: 29298884)
- Klebsiella pneumoniae infections
Infections caused by extreme-drug resistant Gram-negative bacteria may be the greatest emerging threat to immunocompromised and critically ill patients worldwide, and among such pathogens the spread of carbapenem-resistant Enterobacteriaceae (CRE) has become a major concern. The Klebsiella project is working to develop a phage-based therapeutic strategy for reducing the burden of drug-resistant K. pneumoniae in the gastrointestinal tract, and to examine some of the underlying principles that govern the efficacy of such therapies. CPT researchers in collaboration with Weill Cornell Medical College have successfully isolated and characterized panels of virulent phages with activity against K. pneumoniae and developed murine models of K. pneumoniae infection.
- Burkholderia cepacia complex (Bcc) infections in patients with cystic fibrosis (CF)
Bcc infections pose a particular threat to patients with CF due to their high levels of intrinsic resistance to clinically relevant antibiotics. In fact, many clinical isolates are resistant to all currently available antibiotics, rendering effective treatment nearly impossible. There are therefore few or no clinical options to treat infections in the lungs of patients with CF, particularly those with late-stage pulmonary injury. In CF patients, end-stage pulmonary disease often requires lung transplantation. However, pre-transplant colonization with antibiotic-resistant strains of Burkholderia is predictive of poor post-transplant outcomes. Unlike many pathogenic bacteria, Burkholderia has a limited virosphere dominated by temperate (lysogenic) phages that are unsuitable as phage therapeutics. Moreover, the few virulent (obligately lytic) phages that have been isolated have extremely limited host ranges. The CPT is using genetic and molecular approaches to engineer phages for expanded virulence and host range, including rigorous characterization of these phages for their therapeutic characteristics.
Related publications:
Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. (PMID: 20001604)
A Broad-Host-Range Tailocin from Burkholderia cenocepacia. (PMID: 28258146)
Divergence and mosaicism among virulent soil phages of the Burkholderia cepacia complex. (PMID: 16352842)
Modulation of the gut microbiome
Emerging evidence over the past decade has demonstrated the importance of the gut microbiome in a variety of health conditions, including colorectal cancer (CRC), and inflammatory bowel disease (IBD). Certain resident species of the gut microbiome have been linked with initiation and/or maintenance of these health conditions. Given bacteriophage’s narrow host range and natural ability to modulate bacterial populations, phage are uniquely suited to be tools for targeted modulation of the gut microbiome. Antibiotics are ill-suited to this task as their broad killing spectrum makes them likely to kill beneficial bacteria and increase the microbiome dysbiosis that is associated with IBD and CRC. The CPT is working on using virulent bacteriophage for the targeted reduction of IBD-associated sulfate reducing bacteria or cancer-promoting bacteria within the gut. Successful reduction of these target bacteria could form the foundation for novel prevention and treatment of IBD and CRC.
Staphylococcus phages and animal health
Staphylococcus aureus is a major problem in both human and animal health. Our S. aureus project focuses on the diversity and application of S. aureus phage isolated from swine production environments for both swine production purposes as well as possible human applications. Through several grants graciously provided by the National Pork Board starting in 2016, we have sampled 19 swine farms across the United States and isolated over 50 phage from swine production environmental samples. Thirteen of these phage have been tested against a panel of 20 S. aureus strains isolated from both swine and human sources. In addition, select phage isolated during the project were assessed against S. pseudintermedius and S. epidermidis isolates, providing evidence that some of these phages are polyvalent. To date over 20 novel phage genomes have been sequenced and are currently being annotated and compared. The overall goals of this project are to provide insight into the diversity and relatedness of S. aureus phage and to characterize them for their potential therapeutic use.